Chemical synthesis process control system temperature control system TCU
Today, let’s talk about our DCS integrated control system (temperature and other control of synthetic process).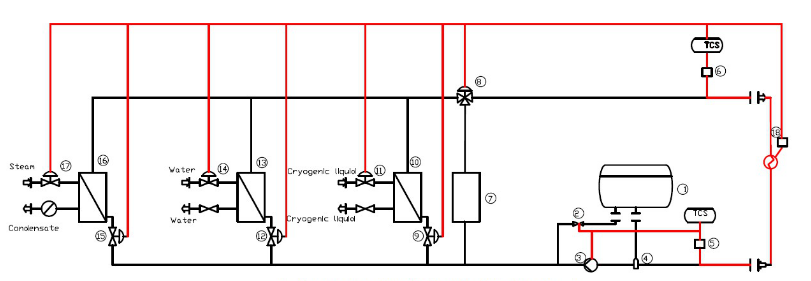
Scalability: the system is an open system, which provides standard TCP / P data communication interface protocol, interface software and application software interface. It has good flexibility and scalability, and meets the requirements of continuously improving production scale for measurement and control ability.
Security: the system has a permission mechanism to realize multi-level permission management. The operating users are divided into five different levels, and the operating permissions of users at different levels are also different. The control system is a relatively closed network, and a firewall is configured between the system network and the enterprise LAN or external network, which can effectively prevent viruses and network attacks and create a safe environment for the operation of the system.
Improve the stability between batches of products: according to the process requirements of pharmaceutical batch production, establish an appropriate production model and strengthen the management of pharmaceutical batches. The batch management of pharmaceutical production is realized through the management modules of production plan designation, production plan release and production plan tracking.
Ensure the integrity of production data: through the application of GMP compliant computer management system and automatic control system, realize the complete automatic collection and monitoring of production data, and carry out effective, accurate and complete data collection, analysis and processing through the computer system, so as to avoid the problems such as lag, missing and error of production data easily caused by the traditional manual recording method.
Improve the traceability of production records: the traceability of pharmaceutical production data is GMP The necessary contents of FDA and other pharmaceutical industry certification, the application of pharmaceutical production automation control system and pharmaceutical intelligent production quality information management system software, real-time, complete and accurate comprehensive records of pharmaceutical production data such as batch, product name, time, production equipment number and operators, and the process of reverse retrieval of production data, which can restore the production status of each production link, Enable producers to analyze the causes of product quality defects, and enable producers to clearly understand the actual production status through the data traceability function, so as to provide data basis for improving production. Technological innovation: realize the real-time monitoring and regulation of key process parameters and quality, clarify the key process control parameters and product characteristics, and then optimize the production process and improve the product quality.
Enhance the market competitiveness. Through the application of advanced pharmaceutical equipment and its automatic control technology, the technical level of Danzhu Dingyan product has greatly improved the product quality and output, and improved the market competitiveness of products.
関連推奨品
-
About the components of the reactor chiller refrigeration heating circulator
1260The reactor chiller refrigeration and heating circulator is used to control the heater and cooler, adjust the temperature, and maintain the stable temperature of the material. Through the circulating medium, the temperature of the material can be ...
詳細を見る -
電気自動車の性能向上のためのバッテリー試験における正確な温度制御
843電気自動車(EV)は自動車業界を完全に変革し、より持続可能でエネルギー効率の高い交通手段を提供している。電気自動車の基本コンポーネントのひとつは、充電可能なバッテリーである。電気自動車...
詳細を見る -
How to solve the problem of poor cooling performance of the heat transfer oil circulation system?
1094Some heat-conducting oil circulation systems will not be as good as before after 3 months of use. At this time, it is recommended to contact the professional after-sales department to check the user's power supply and whether the equipment is norm...
詳細を見る -
2019 CPHl China | LNEYA W5P22 Exhibition Site&Wonderful
1050From June 18th to June 20th, 2019, the 19th World Pharmaceutical Raw Materials China Exhibition - CPhI China 2019 was held at the Shanghai New International Expo Center. LNEYA took a variety of equipment to the W5P22 booth, which lasted for three ...
詳細を見る
 LNEYA工業用冷凍機 メーカー サプライヤー
LNEYA工業用冷凍機 メーカー サプライヤー













